What is a Water Softener?
What is a Water Softener?
What is a Water Softener?
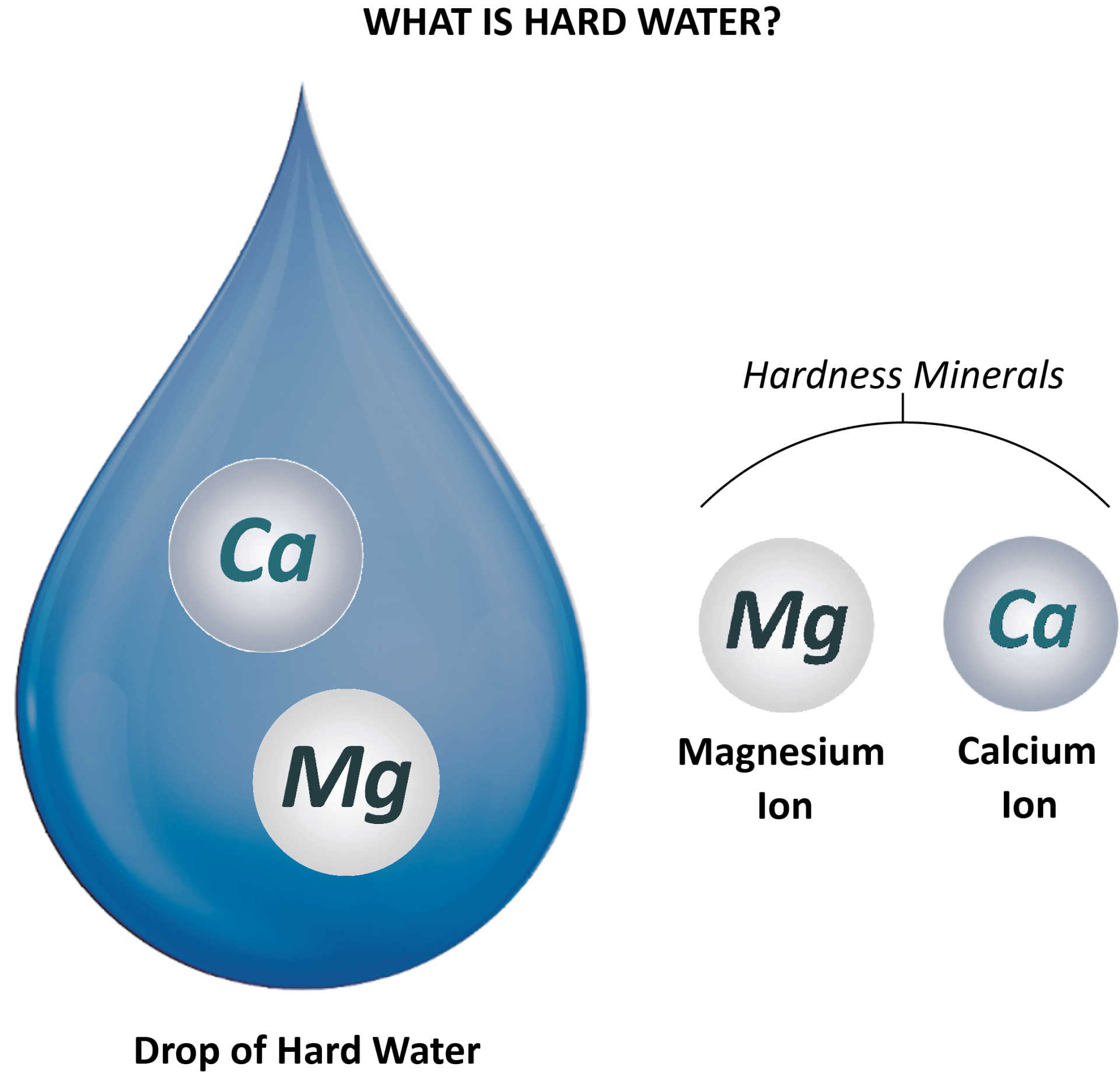
A water softener is an appliance which removes hardness minerals (such as chalk and lime) from mains water.
It is connected directly onto the incoming water main to a property and removes all of the hard minerals (magnesium
and calcium) in the water — thus producing softened water.
By removing these hard water minerals, you will be scale free and can enjoy numerous benefits all around the home.
These benefits don’t just include the ones you can see and feel, but also the ones you cannot see like your internal pipework and appliances.
By eradicating scale and scum, you and your home will reap the benefits in all areas.
You will wish you had installed one sooner!
How does a Water Softener work?
A water softener is an appliance which removes hardness minerals from mains water.
It is connected directly on to the incoming water main and through a process called ion-exchange, it removes the magnesium and calcium minerals in the water — thus producing softened water.
Ion-exchange is the most thorough
and cost effective
way to produce
softened water and is the method used in all water softener units.
What is Ion-Exchange?
Simply put, ion-exchange is a chemical process where certain ions are exchanged. This procedure is carried out with the use of ion exchange resin.
The resin is made of tiny polymeric beads which have the ability to be charged with certain ions and then swap with others.
In the case of water softening, the purpose of the process is to exchange the unwanted dissolved ions (magnesium & calcium) in the water.
This is what the resin beads look like...
How does the ion-exchange process work?
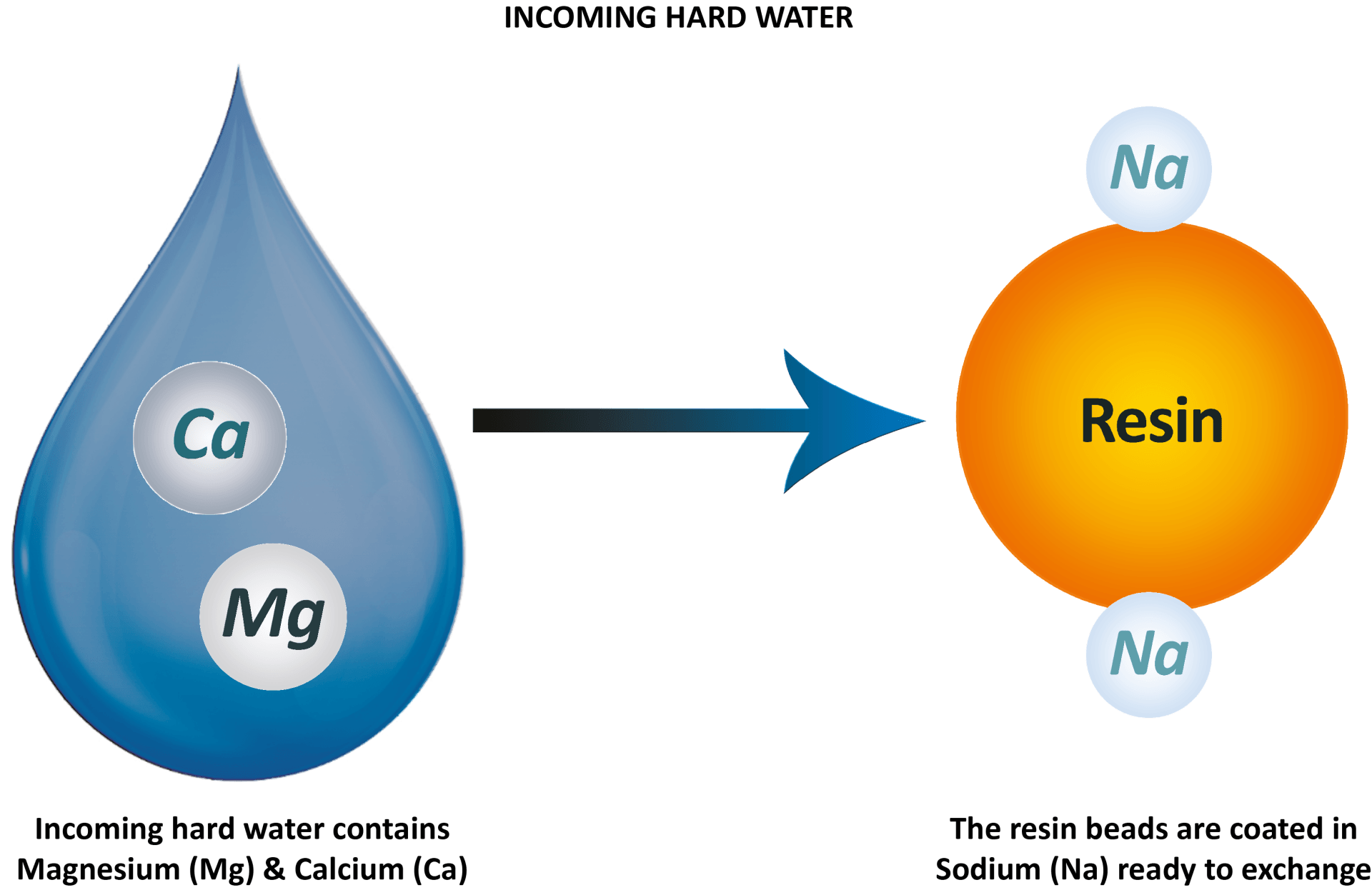
Incoming hard water contains hardness minerals - magnesium and calcium
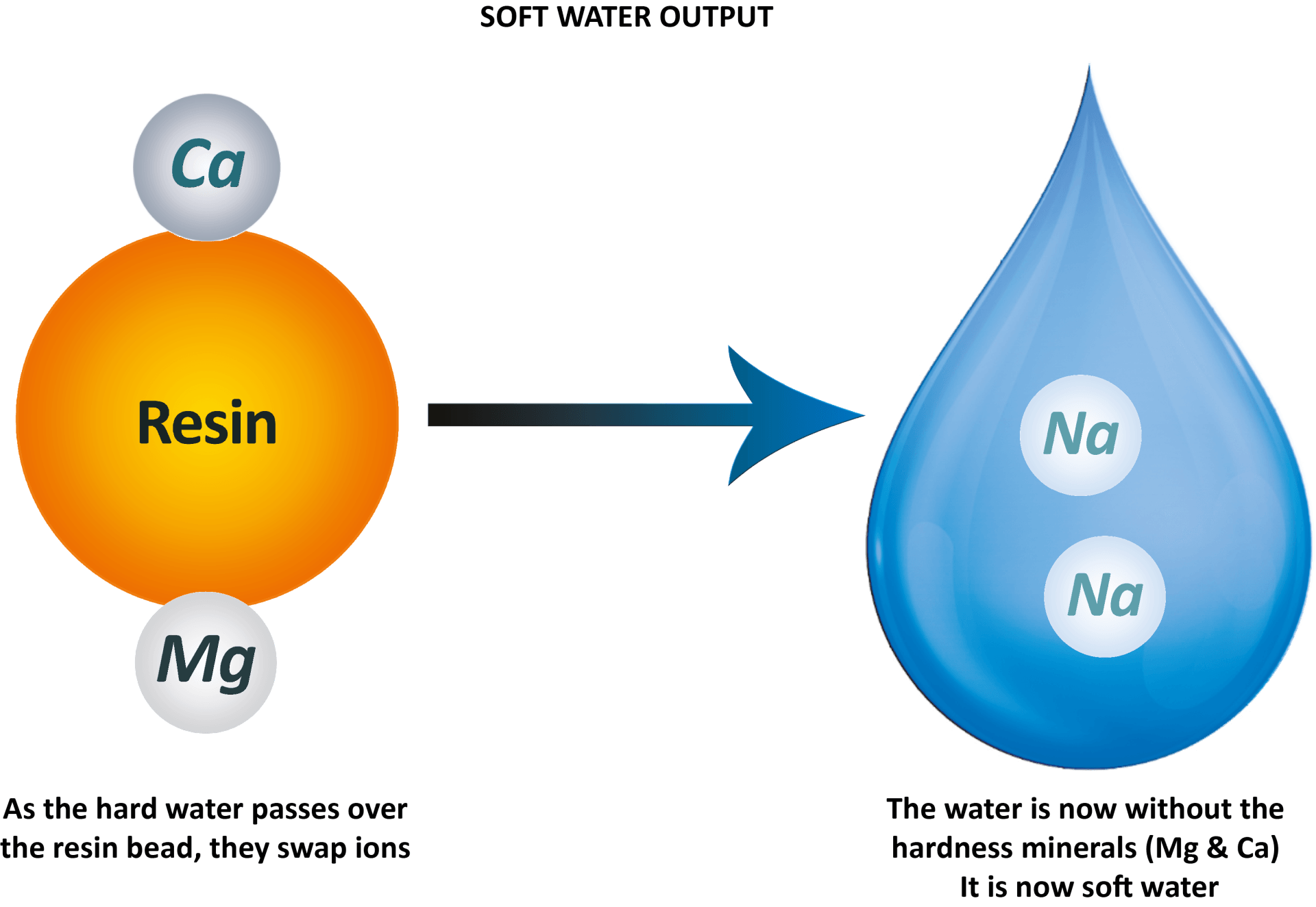
- in every drop. The resin beads inside the cylinders are coated in sodium ions ready to exchange for the unwanted magnesium and calcium in the water. As the drop of hard water comes into contact with the resin bead, they switch ions.
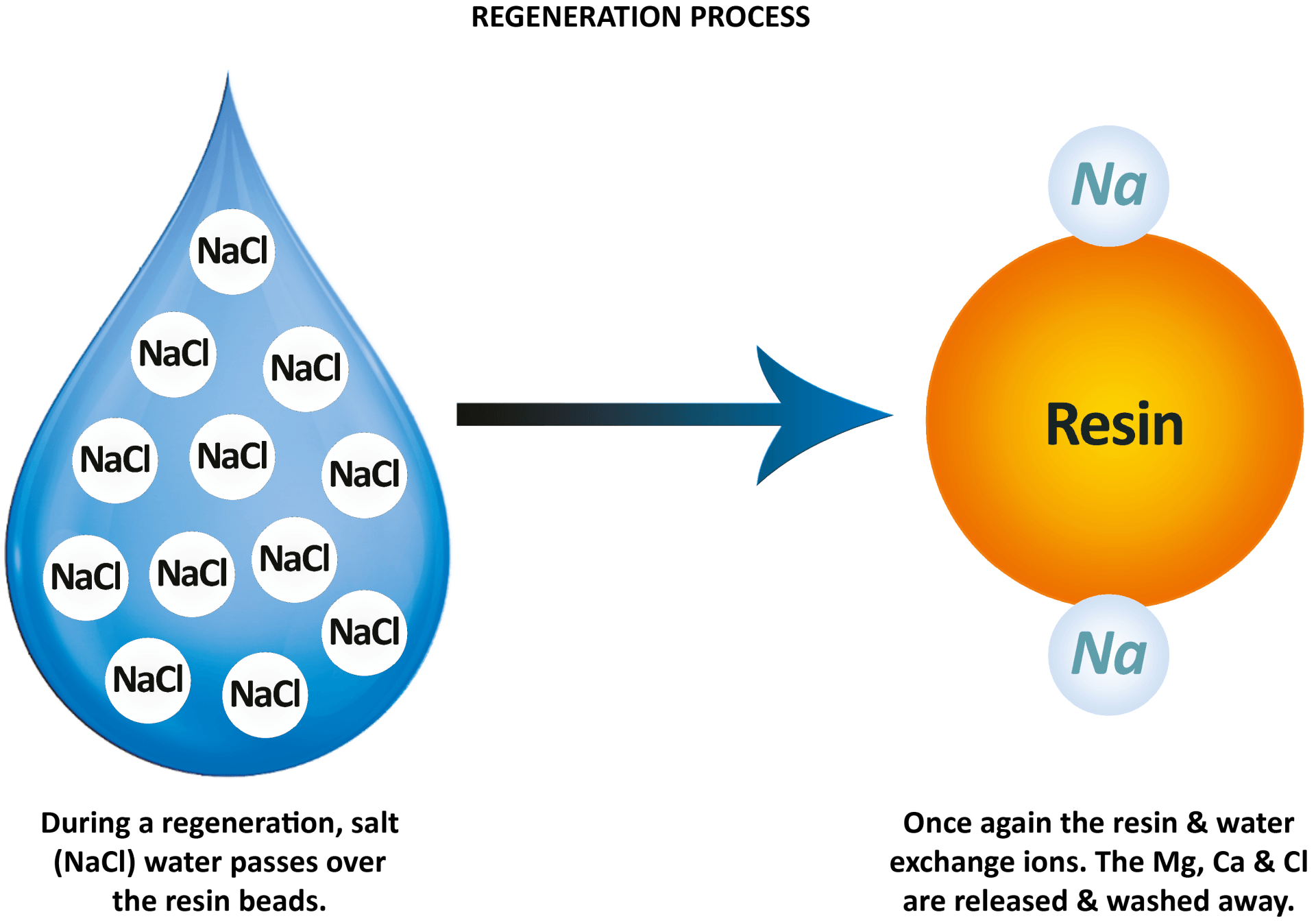
What happens during a regeneration?
The regeneration process is basically a cleaning cycle for the resin. Once the resin has swapped as many magnesium and calcium ions as it can through ion-exchange, it will need to be flushed to recharge/clean. At this point the cylinder stops its operation temporarily ready to clean.
To recharge the resin it needs to be flushed with a salt solution — (hence why you put salt in a water softener).
By recharging the beads with a coating of sodium ions, it means they are activated again so the water softener can repeat the process and remove more magnesium and calcium ions from the incoming hard water.
How long does a regeneration cycle take?
This process varies from softener to softener - some can take 40-90+ minutes!
However, with one of our water softeners the entire process is just 11 minutes.
We have the whole of East Anglia covered with our 3 showrooms...
IPSWICH
TwinTec House
150 Spring Road
Ipswich
IP4 5NR
PHONE
01473 713600
SAXMUNDHAM
Infinity House
6 South Entrance
Saxmundham
IP17 1DQ
PHONE
01728 633007
BRAINTREE
295 Coggeshall Road,
Braintree
Essex
CM7 9ED
PHONE
01376 334949
Powered by LocaliQ